Search
Showing results for "clinical trials"
The aim of this study was to understand the challenges experienced by families obtaining a diagnosis and therapy for developmental coordination disorder (DCD). Parents of 435 children aged 4-18 years with persistent motor difficulties consistent with a diagnosis of DCD completed an online survey. Diagnostic timeline and diagnostic label/s received were examined, along with therapies accessed.
Review of the clinical and biological progress over 50 years in Rett Syndrome
Clinical presentations and genetic variations in Cyclin-dependent kinase-like 5 deficiency disorder based on a systematic literature review and experience
Clinical decision support systems (CDSS) are increasingly utilised within healthcare settings to enhance decision making. However, few studies have investigated their application in the context of clinical services for autistic people, with no research to date exploring the perspectives of the key stakeholders who are, or in the future may be, impacted by their use.
MEDIA ENQUIRIES Discover. Prevent. Cure. Mailing list Media contacts About The Kids Be Inspired Please direct general enquiries to our reception on (
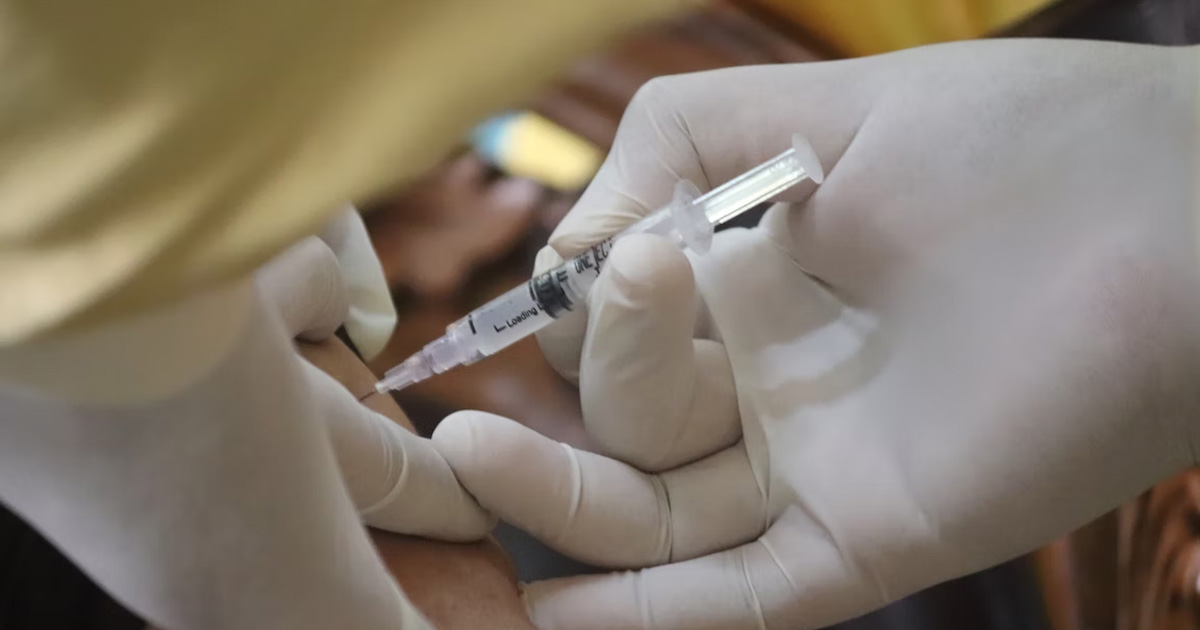
Influenza and COVID-19 infections during pregnancy may have serious adverse consequences for women as well as their infants. However, uptake of influenza and COVID-19 vaccines during pregnancy remains suboptimal. This study aims to assess the effectiveness of a multi-component nudge intervention to improve influenza and COVID-19 vaccine uptake among pregnant women.
Perioperative respiratory adverse events pose a significant risk in pediatric anesthesia, and identifying these risks is vital. Traditionally, this is assessed using history and examination. However, the perioperative risk is multifactorial, and children with complex medical backgrounds such as chronic lung disease or obesity may benefit from additional objective preoperative pulmonary function tests.

Epigenomic research at The Kids explores the links between childhood disease and the molecular hallmarks of epigenetic control.
The main aim of the study was to ascertain HPV-16/18 vaccine efficacy in both full and naive cohorts and to explore protection conferred against non-vaccine HPV
Prioritisation of clinical trials ensures that the research conducted meets the needs of stakeholders, makes the best use of resources and avoids duplication. The aim of this review was to identify and critically appraise approaches to research prioritisation applicable to clinical trials, to inform best practice guidelines for clinical trial networks and funders.
