Search
The evolutionary acquisition of mitochondria has given rise to the diversity of eukaryotic life. Mitochondria have retained their ancestral α-proteobacterial traits through the maintenance of double membranes and their own circular genome. Their genome varies in size from very large in plants to the smallest in animals and their parasites. The mitochondrial genome encodes essential genes for protein synthesis and has to coordinate its expression with the nuclear genome from which it sources most of the proteins required for mitochondrial biogenesis and function.
Changes in the rate and fidelity of mitochondrial protein synthesis impact the metabolic and physiological roles of mitochondria. Here we explored how environmental stress in the form of a high-fat diet modulates mitochondrial translation and affects lifespan in mutant mice with error-prone or hyper-accurate mitochondrial ribosomes. Intriguingly, although both mutations are metabolically beneficial in reducing body weight, decreasing circulating insulin and increasing glucose tolerance during a high-fat diet, they manifest divergent (either deleterious or beneficial) outcomes in a tissue-specific manner.
Transcriptomic technologies have revolutionized the study of gene expression and RNA biology. Different RNA sequencing methods enable the analyses of diverse species of transcripts, including their abundance, processing, stability, and other specific features. Mitochondrial transcriptomics has benefited from these technologies that have revealed the surprising complexity of its RNAs. Here we describe a method based upon cyclization of mitochondrial RNAs and next generation sequencing to analyze the steady-state levels and sizes of mitochondrial RNAs, their degradation products, as well as their processing intermediates by capturing both 5' and 3' ends of transcripts.
The genetic code that specifies the identity of amino acids incorporated into proteins during protein synthesis is almost universally conserved. Mitochondrial genomes feature deviations from the standard genetic code, including the reassignment of two arginine codons to stop codons.
Mitophagy preserves overall mitochondrial fitness by selectively targeting damaged mitochondria for degradation. The regulatory mechanisms that prevent PTEN-induced putative kinase 1 (PINK1) and E3 ubiquitin ligase Parkin (PINK1/Parkin)-dependent mitophagy and other selective autophagy pathways from overreacting while ensuring swift progression once initiated are largely elusive.
The number of tRNA isodecoders has increased dramatically in mammals, but the specific molecular and physiological reasons for this expansion remain elusive. To address this fundamental question we used CRISPR editing to knockout the seven-membered phenylalanine tRNA gene family in mice, both individually and combinatorially.
The discovery of CRISPR-Cas9 and its widespread use has revolutionised and propelled research in biological sciences.
BNIP3 and NIX are the main receptors for mitophagy, but their mechanisms of action remain elusive. Here, we used correlative light EM (CLEM) and electron tomography to reveal the tight attachment of isolation membranes (IMs) to mitochondrial protrusions, often connected with ER via thin tubular and/or linear structures.
Directed evolution emulates the process of natural selection to produce proteins with improved or altered functions. These approaches have proven to be very powerful but are technically challenging and particularly time and resource intensive. To bypass these limitations, we constructed a system to perform the entire process of directed evolution in silico.
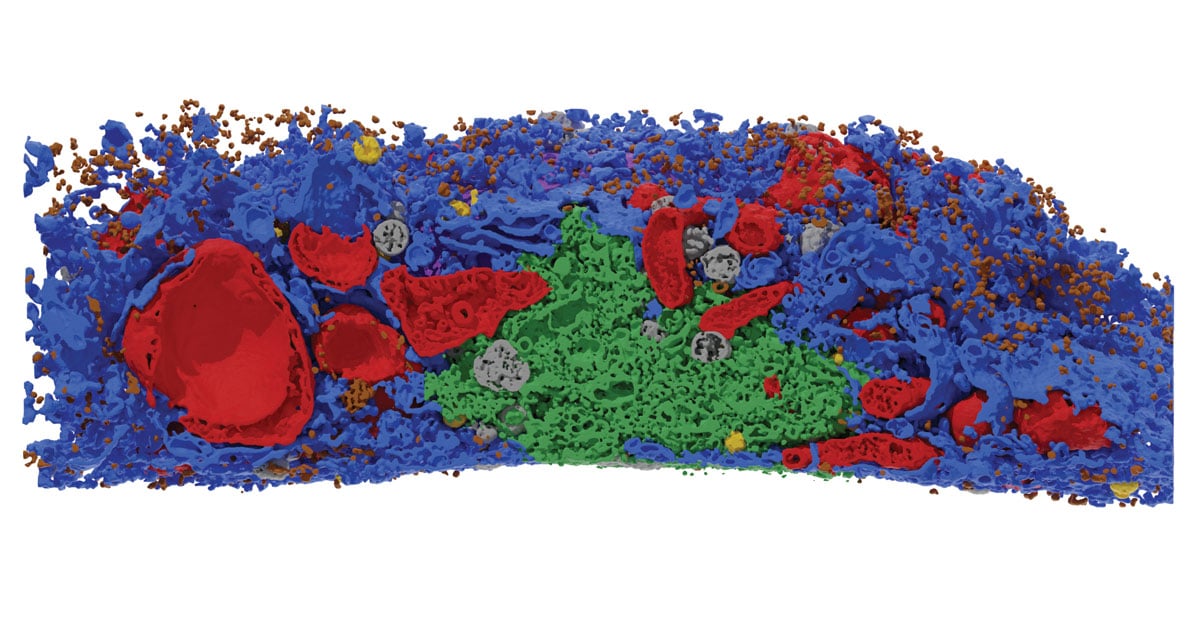
Researchers from The Kids Research Institute Australia and The University of Western Australia have developed a new technique to see inside cells with unprecedented detail, revealing a complicated web of interactions that provides new insights into how cells stay healthy.
